- October 19 + 20, 2022, the CDC (Centers for Disease Control) held a conference to update policies since last meeting was 2 years ago, with approval to include Covid-19 vaccination guidelines for 2023.
- The streamlined agenda gave no deviation for contrary opinions to the presentation.
- One CDC outsider was given the opportunity to express an opinion: This vaccination injured person, appealed to cease the CDC vote, but was quicky informed to end their dialogue.
- The meeting was a formality to execute their agenda: “Approval”
- The Recommended Child and Adolescent Immunization Schedule, United States, 2023 and the Recommended Adult Immunization Schedule, United States, 2023
- The presented research was not from non-biased Third-Party, but by the very same drug manufacturers supplying the drugs.
- The focus of concern:
- Cost benefit analysis
- Insurance coverage costs
- Assessing Population, age, health factors, ethnicity for potential risks and reduce outbreaks

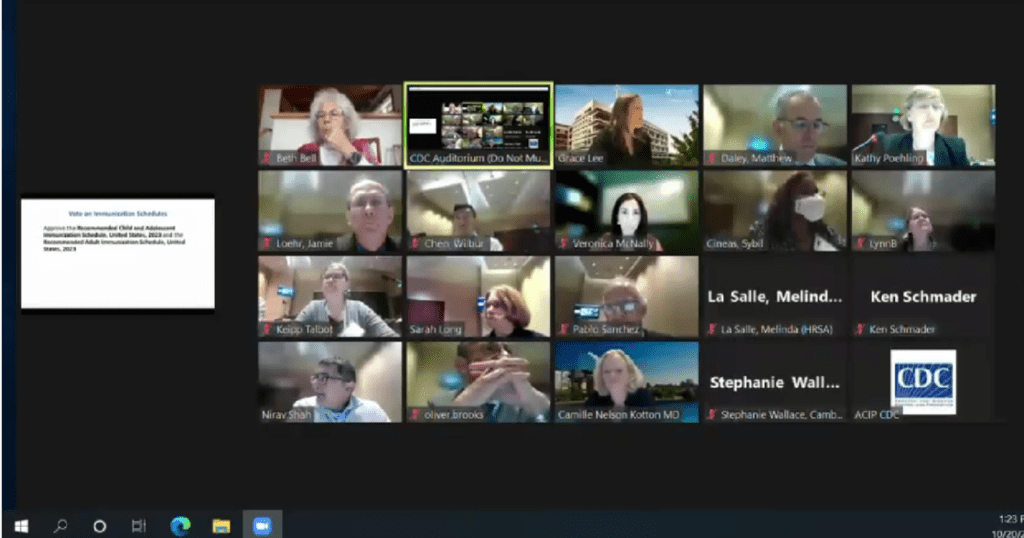
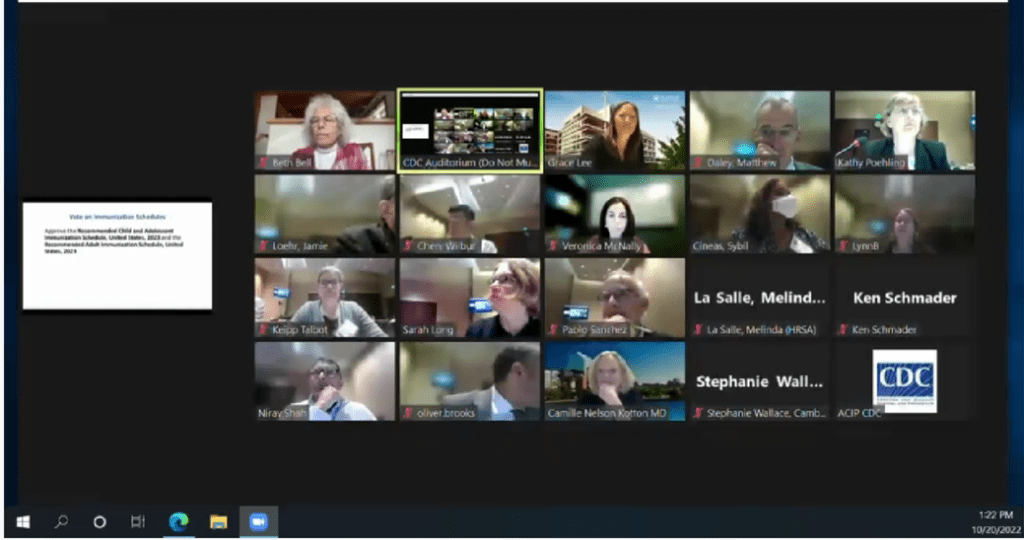
CDC Panel, voted unanimous “Approval:” The photo features those (link to their bios on CDC site) as follows: Beth Bell, Grace Lee, Matthew Daley, Kathy Poehling, Veronica McNally, Sybil Cineas, Lynn B, Jamie Loehr, Wilber Chen, Helen Keipp Talbot, Sarah Long, Pablo Sanchez, Melina LaSalle (HRSA), Ken Schmader, Nirav Shah, Oliver Brooks, Camille Nelson Kotton, MD, Stephanie Wallace (Cambridge)
CDC Membership Roster with member background: https://www.cdc.gov/vaccines/acip/members/bios.html